Current location:Home > Product > Raw material of cosmeticss

|
Hazard |
Moderately toxic. |
|
Safety Profile |
Moderarely toxic by intraperitoneal route. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and fumes. See also COUMARIN |
|
Application |
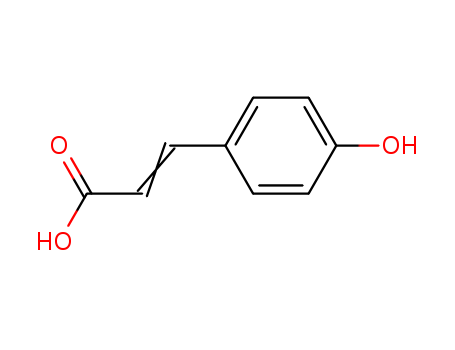
p-Hydroxycinnamic acid is mainly used as an intermediate in the pharmaceutical and perfume industries. It can react with dimethyl sulfate to produce p-anisaldehyde, react with acetaldehyde to produce p-hydroxycinnamaldehyde, and further oxidize it to produce cinnamic acid. This product can be directly oxidized to produce 4-Hydroxybenzoic acid and reduction to produce 4-Hydroxybenzyl alcohol. |
|
Definition |
ChEBI: 4-coumaric acid is a coumaric acid in which the hydroxy substituent is located at C-4 of the phenyl ring. It has a role as a plant metabolite. It is a conjugate acid of a 4-coumarate. |
InChI:InChI=1/C9H8O3/c1-6(9(11)12)7-2-4-8(10)5-3-7/h2-5,10H,1H2,(H,11,12)
The Fujiwara-Moritani reaction has had a...
The number of deaths or critical health ...
Herein, a dual nickel/ruthenium strategy...
Bioassays guided phytochemical investiga...
![cyanidin 3-O-[2-O-(β-xylopyranosyl)-6-O-(4-O-(6-O-(trans-feruloyl)-β-glucopyranosyl)-trans-p-coumaroyl)-β-glucopyranoside]-5-O-[6-O-(malonyl)-β-glucopyranoside]](/upload/2025/11/e5bb635f-76d3-4f96-a160-8e783972df9a.png)
cyanidin 3-O-[2-O-(β-xylopyranosyl)-6-O-(4-O-(6-O-(trans-feruloyl)-β-glucopyranosyl)-trans-p-coumaroyl)-β-glucopyranoside]-5-O-[6-O-(malonyl)-β-glucopyranoside]

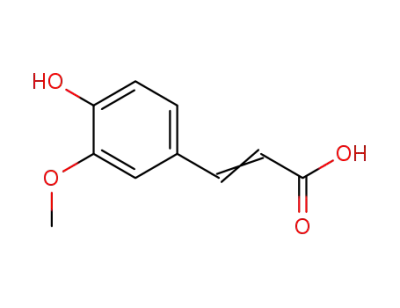
3-(4-hydroxy-3-methoxyphenyl)acrylic acid

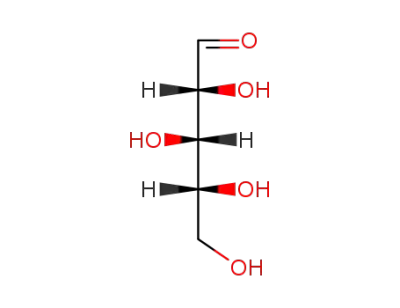
D-xylose

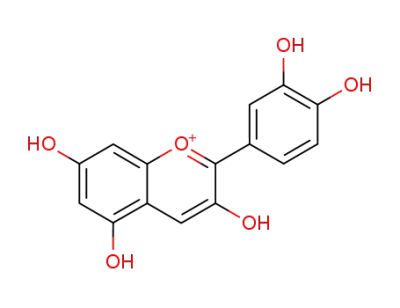
cyanidin

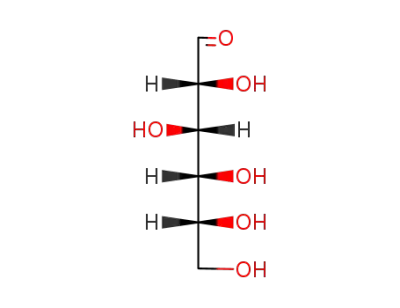
D-glucose

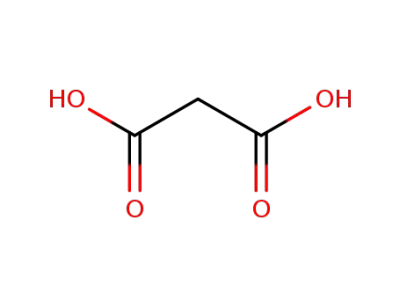
malonic acid

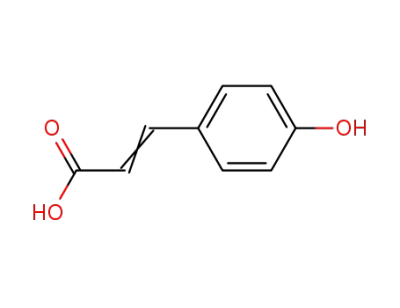
para-coumaric acid
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
at 100 ℃;
for 2h;
|
![cyanidin 3-O-[2-O-(2-O-(trans-feruloyl)-β-xylopyranosyl)-6-O-(4-O-(β-glucopyranosyl)-trans-pcoumaroyl)-β-glucopyranoside]-5-O-[6-O-(malonyl)-β-glucopyranoside]](/upload/2025/11/c8d0dd43-1113-4367-b34d-e65665c7a4ca.png)
cyanidin 3-O-[2-O-(2-O-(trans-feruloyl)-β-xylopyranosyl)-6-O-(4-O-(β-glucopyranosyl)-trans-pcoumaroyl)-β-glucopyranoside]-5-O-[6-O-(malonyl)-β-glucopyranoside]


3-(4-hydroxy-3-methoxyphenyl)acrylic acid


D-xylose


cyanidin


D-glucose


malonic acid


para-coumaric acid
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
at 100 ℃;
for 2h;
|
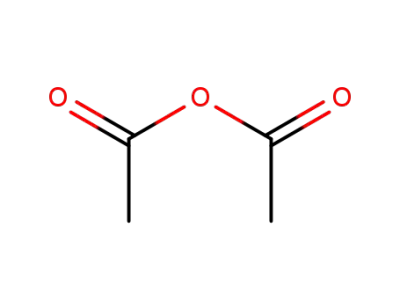
acetic anhydride
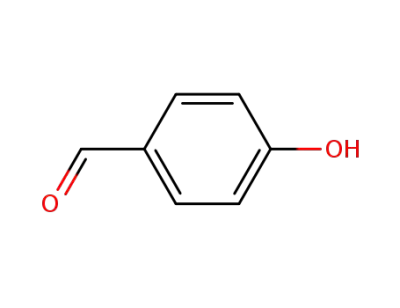
4-hydroxy-benzaldehyde

malonic acid
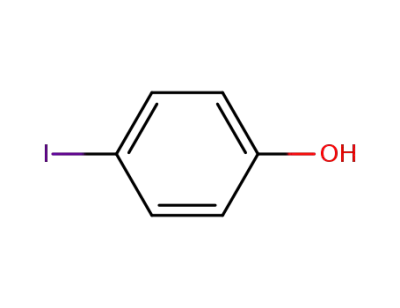
p-Iodophenol
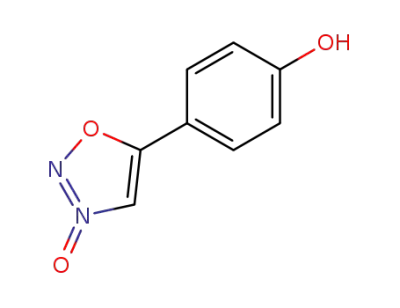
4-(3-oxy-[1,2,3]oxadiazol-5-yl)-phenol
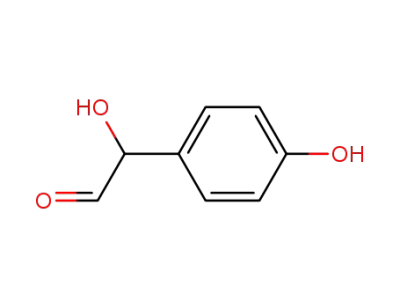
hydroxy-(4-hydroxy-phenyl)-acetaldehyde
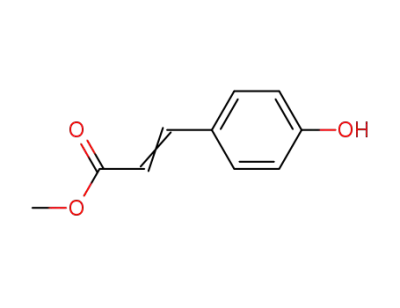
methyl p-hydroxycinnamate
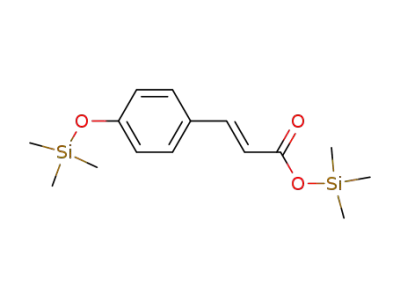
4-trimethylsiloxycinnamic acid