Current location:Home > Product > Fire retardant

|
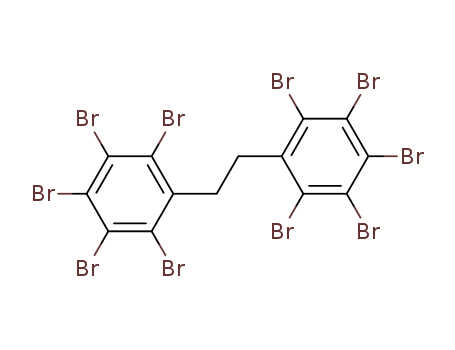
Physical properties |
White or pale yellow powder. Slightly soluble in alcohol, ether, almost insoluble in water. |
|
Preparation |
Diphenyl ethane (DPE) was synthesized by using benzene and dichloroethane as raw materials and AlC3 as catalyst, and then DPE was brominated and aged to obtain decabromodiphenyl ethane. |
|
Application |
Decabromodiphenyl ethane, a widely used new brominated flame retardant, is added into flammable materials to achieve fire retardation. It used in thermoplastics, thermosets, textiles and coatings that inhibit or resist the spread of fire. |
|
Environmental Fate |
Decabromodiphenyl ethane’s physicochemical properties suggest that it will partition predominantly in sediment and soil through binding to the organic fraction of particulate matter. In the EA environmental risk assessment, it concluded that DBDPEthane is unlikely to rapidly undergo photodegradation in the presence of hydroxyl radicals, it is not readily biodegradable in the aquatic environment under aerobic conditions, and it is not hydrolysable (no hydrolysable groups) (Dungey and Akintoye, 2007). No data were available to assess the biodegradation under anaerobic conditions [e.g., wastewater treatment plants (WWTPs) or in sediments]; the EA noted the possibility of reductive debromination under these conditions. The potential for DBDPEthane to bioconcentrate was evaluated in an 8-week fish bioconcentration study (Dungey and Akintoye, 2007). When fish were exposed to DBDPEthane at concentrations of 0.5 and 0.05mg/l, the respective fish bioconcentration factors (BCFs) were <2.5 and <25 l/kg wet weight (ww), respectively. Since the water concentrations used exceeded DBDPEthane’s water solubility, the dissolved concentration was unknown. The EA appropriately considered this study invalid. No additional BCF studies are available; however, based on the physicochemical properties, lowtoxicity in acute and repeated dose mammalian, aquatic, and terrestrial studies, it is unlikely that DBDPEthane will bioaccumulate. |
InChI:InChI=1/C14H4Br10/c15-5-3(6(16)10(20)13(23)9(5)19)1-2-4-7(17)11(21)14(24)12(22)8(4)18/h1-2H2
A high assay decabromodiphenylalkane pro...
Bromine is scrubbed from a gaseous mixtu...
High assay, reaction-derived decabromodi...
High assay reaction-derived decabromodip...

1,1'-(1,2-ethanediyl)bisbenzene

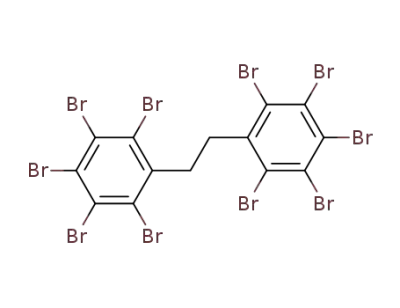
1,2-bis(pentabromophenyl)ethane
| Conditions | Yield |
|---|---|
|
With
bromine;
aluminum tri-bromide;
In
1,1-dibromomethane;
at 73 - 98 ℃;
Product distribution / selectivity;
|
95.5% |
|
1,1'-(1,2-ethanediyl)bisbenzene;
With
hydrogen bromide; bromine;
iron(III) chloride;
at 56 - 66 ℃;
for 3.2 - 5h;
With
bromine;
aluminum (III) chloride;
at 57.3 - 59.7 ℃;
for 4.48333 - 5.58333h;
Product distribution / selectivity;
Heating / reflux;
|
|
|
1,1'-(1,2-ethanediyl)bisbenzene;
With
hydrogen bromide; bromine;
iron(III) chloride;
at 60 - 80 ℃;
for 3.76667h;
With
bromine;
aluminum (III) chloride; ferric(III) bromide;
at 58.1 - 58.2 ℃;
for 3.36667h;
Product distribution / selectivity;
Heating / reflux;
|
|
|
With
bromine;
aluminum (III) chloride;
In
1,1-dibromomethane;
at 55 ℃;
for 4.08 - 8.5h;
Product distribution / selectivity;
Heating / reflux;
|
|
|
With
hydrogen bromide; bromine;
aluminum (III) chloride;
In
1,1-dibromomethane;
at 60 - 86.9 ℃;
for 1.18333 - 7.95h;
under 2156.39 - 2880.41 Torr;
Product distribution / selectivity;
|
|
|
With
bromine;
aluminum (III) chloride;
In
1,1-dibromomethane;
at 55.1 - 64.2 ℃;
for 1.96667 - 2h;
Product distribution / selectivity;
Heating / reflux;
|

2,3,4,5,6-pentabromobenzyl bromide

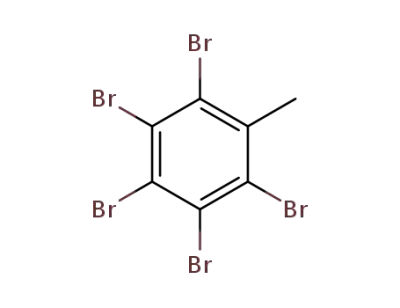
pentabromotoluene


1,2-bis(pentabromophenyl)ethane
| Conditions | Yield |
|---|---|
|
With
2,4,6-triphenyl-2H-pyran;
In
isopropyl alcohol;
for 2h;
Heating;
|
9% 7% |

2,3,4,5,6-pentabromobenzyl bromide

1,1'-(1,2-ethanediyl)bisbenzene