Current location:Home > Product > Vitamins and amino acids

|
Definition |
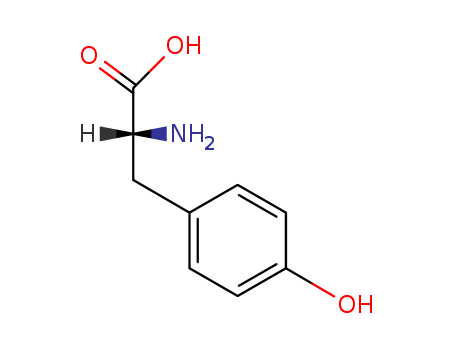
ChEBI: An optically active form of tyrosine having D-configuration. |
InChI:InChI=1/C9H11NO3/c10-8(9(12)13)5-6-1-3-7(11)4-2-6/h1-4,8,11H,5,10H2,(H,12,13)/t8-/m1/s1
Separation of racemic mixtures is of gre...
l-Homoalanine, a useful building block f...
(S)-2-Aminobutane, d-alanine, and d-homo...
The conversion of aldehydes and ketones ...
N-((Ξ)-benzylidene)-L-tyrosine ; sodium-salt

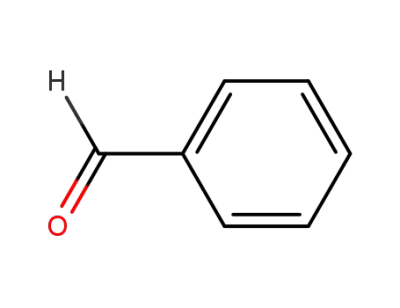
benzaldehyde

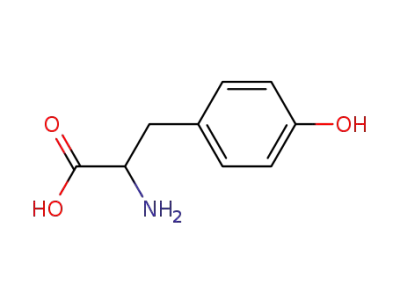
?Tyr
| Conditions | Yield |
|---|---|
|
|
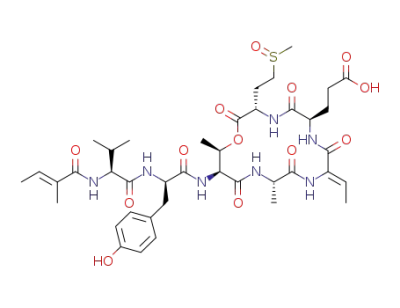
tiglicamide C

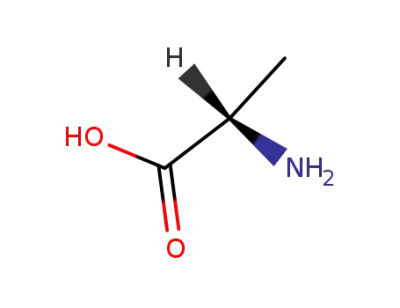
L-alanin

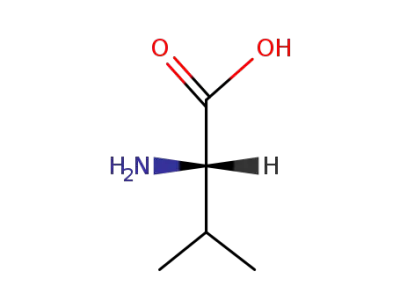
L-valine

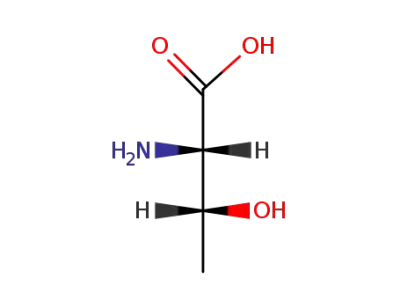
L-threonine

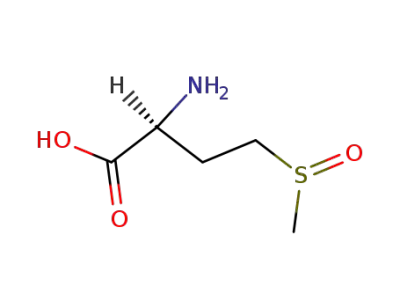
L-methionine sulfoxide

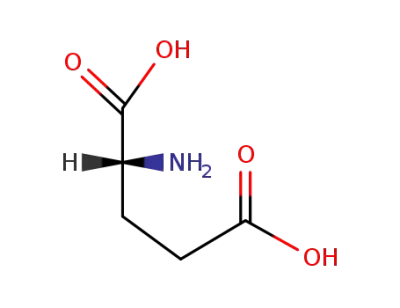
D-Glutamic acid

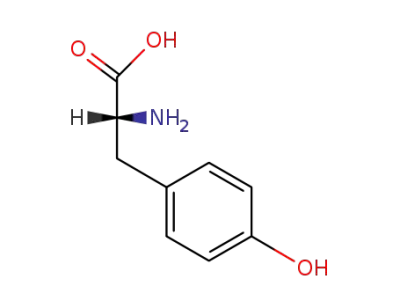
tyrosine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
at 116 ℃;
for 24h;
|
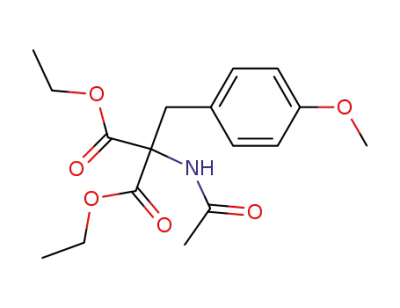
2-acetylamino-2-(4-methoxybenzyl)malonic acid diethyl ester
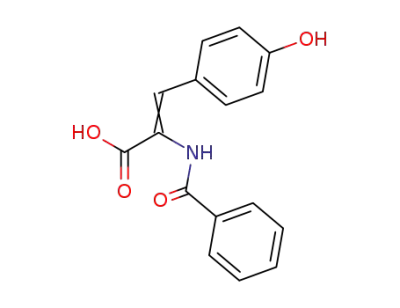
2-benzoylamino-3-(4-hydroxy-phenyl)-acrylic acid
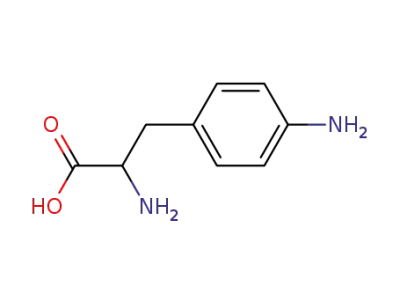
(p-aminophenyl)alanine
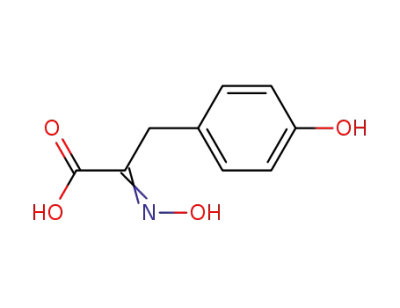
[2-hydroxyimino-3-(4-hydroxyphenyl)]propionic acid
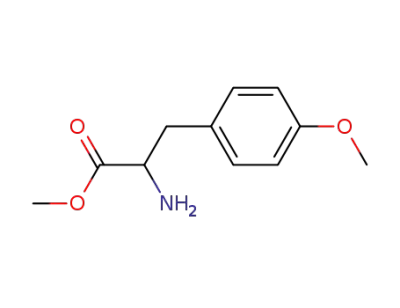
methyl 2-amino-3-(4-methoxyphenyl) propanoate
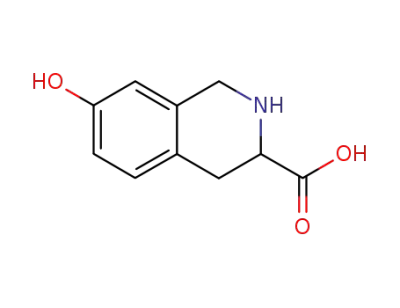
7-hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid

tyrosine isopentyl ester
N-carbamoyltyrosine