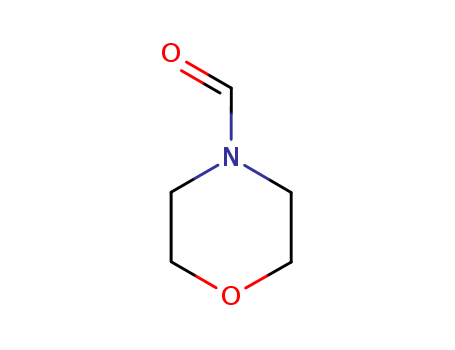

|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 25, p. 199, 1960 DOI: 10.1021/jo01072a012 |
|
Flammability and Explosibility |
Nonflammable |
|
General Description |
Treatment of 4-formylmorpholine with sulphur tetrafluoride in the presence of potassium fluoride gives 4-(trifluoromethyl)morpholine in excellent yields. 4-Formylmorpholine reacts with series of 2-alkyl-2-cyclohexen-1-ones in the presence of POCl3 to give the corresponding 3-alkyl-2-chloro-5,6-dihydrobenzaldehydes and allylic alcohols (by-product). |
InChI:InChI=1/C5H9NO2/c7-5-6-1-3-8-4-2-6/h5H,1-4H2
The elusive 4-methyl-3,4-dihydro-2H-[1,4...
-
-
Formic acid is used as the sole carbon a...
Bis-NHC stabilized germyliumylidenes [RG...
-
A nickel-catalyzed amination of aryl chl...
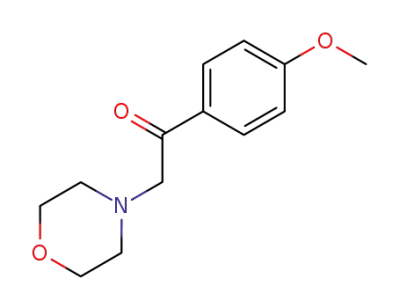
1-(4-methoxyphenyl)-2-(4-morpholinyl)ethanone

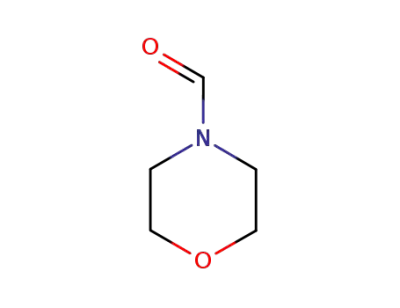
4-morpholinecarboxaldehyde

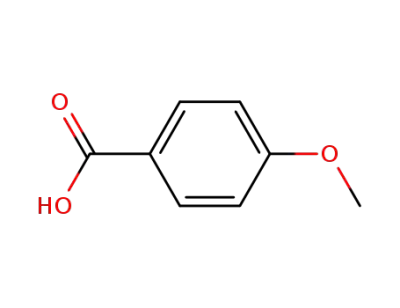
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With
TEMPO; oxygen;
In
acetonitrile;
at 50 ℃;
for 38h;
under 760.051 Torr;
|
50% 73% |
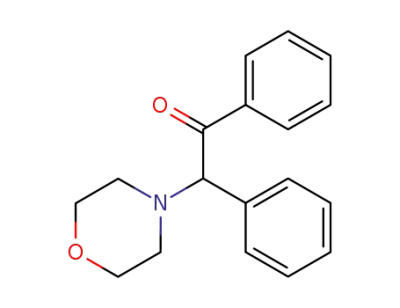
α-(N-morpholino)-α-phenylacetophenone

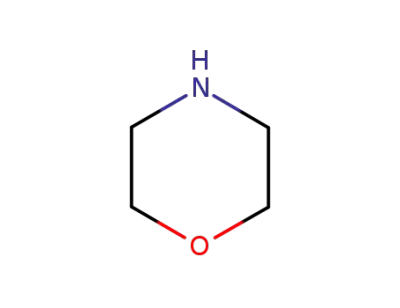
morpholine


4-morpholinecarboxaldehyde

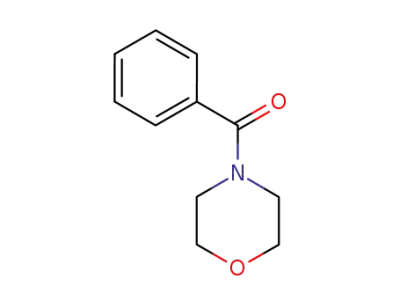
4-benzoylmorpholine

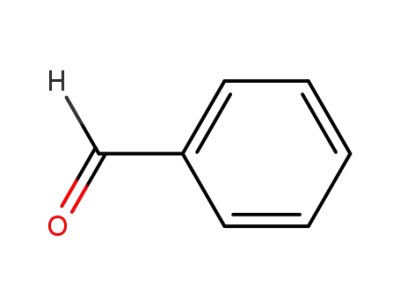
benzaldehyde

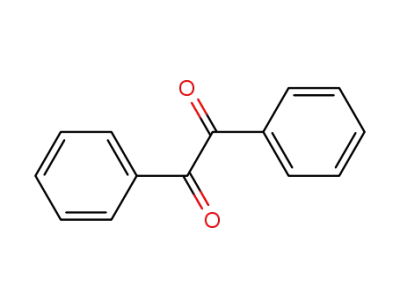
benzil

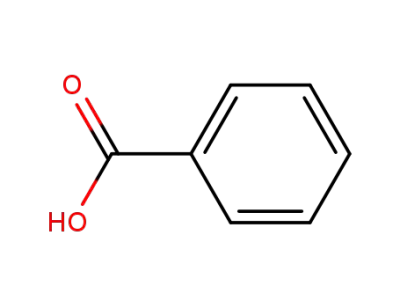
benzoic acid
| Conditions | Yield |
|---|---|
|
With
oxygen; rose bengal;
In
water; benzene;
Quantum yield;
Irradiation;
oxidative fragmentation;
|
56 % Chromat. 12 % Chromat. 10 % Chromat. 10 % Chromat. |

morpholine
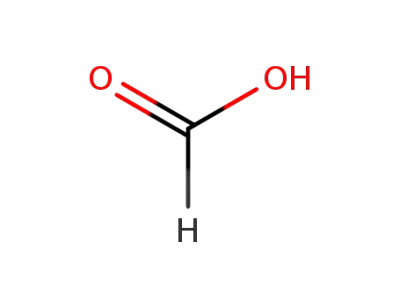
formic acid

hydrogen cyanide
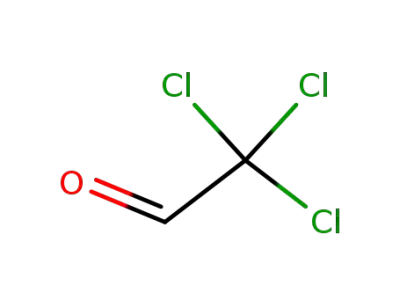
chloral
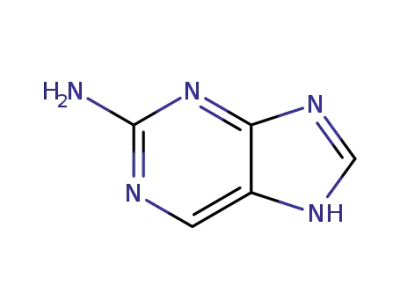
7H-purin-2-amine
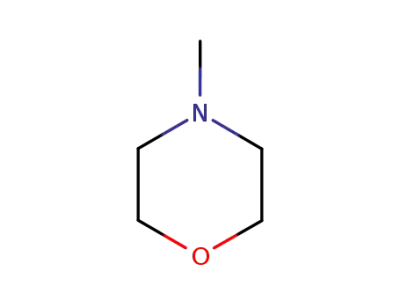
4-methyl-morpholine
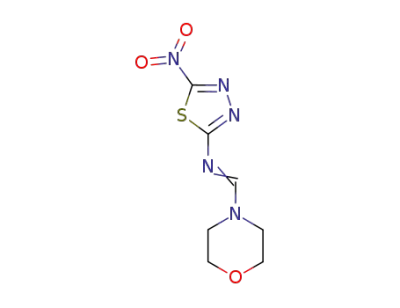
4-[N-(5-nitro-[1,3,4]thiadiazol-2-yl)-formimidoyl]-morpholine
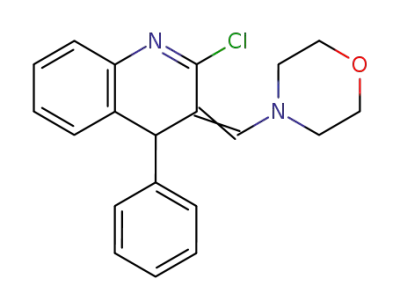
2-chloro-3-morpholin-4-ylmethylene-4-phenyl-3,4-dihydro-quinoline