Current location:Home > Product > Plasticiser

|
General Description |
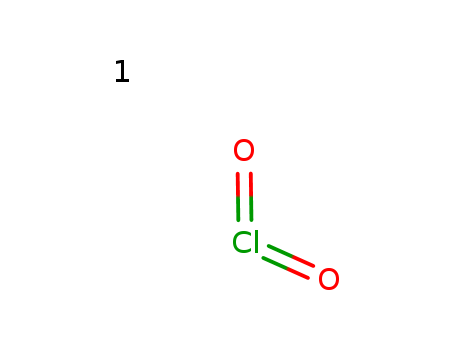
Chlorine dioxide is a yellow-green gas with a pungent odor that is commonly used as a disinfectant and bleaching agent. It is highly effective at killing bacteria, viruses, and other microorganisms, making it a popular choice for water treatment and sanitization in various industries. Chlorine dioxide is also used in the food and beverage industry to sanitize food processing equipment and to help preserve food products. It is considered to be a safer alternative to chlorine for disinfection as it produces fewer harmful disinfection byproducts. However, it is important to handle chlorine dioxide with caution as it is a strong oxidizing agent and can be hazardous if not used properly. |
InChI:InChI=1/ClO2/c2-1-3
Dispersed laser-induced fluorescence of ...
The primary quantum yields of O(3PJ) and...
Second-order rate constants for the deca...
The kinetics of several redox reactions ...
Ozone reactions with XO2- (X = Cl or Br)...
-
The overall rate constant of the BrO + C...
The jet-cooled A(2A2) (2B1) absorption s...
Photofragment translational energy spect...
The excited electronic state of OClO in ...
Velocity map imaging was used to study t...
The flash photolysis-ultraviolet absorpt...
Chlorine dioxide, an industrially import...
The initial rate of formation of chlorin...
The reaction between ClO2- and HOCl has ...
The stoichiometry and kinetics of the re...
The effect of chloride ion on the chlori...
Kinetics for reactions of OBrO with NO, ...
Reaction between chlorine(III) and bromi...
A water-soluble manganese porphyrin comp...
Chloride oxyanions are of interest becau...
The kinetics and mechanism of the chlori...
The ultraviolet and infrared absorption ...
The reaction between chlorite and the am...
The oxidation of L-cysteine and its meta...
HOOClO2 was produced by addition of hydr...
The chlorite-tetrathionate reaction has ...
The rate constant for the radical-radica...
The photochemistry of chlorine dioxide (...
We report a series of time-resolved infr...
Coherent anti-Stokes Raman scattering (C...
The reaction kinetics for the reaction O...
The kinetics and mechanism of the chlori...
The UV photolysis of Cl2O2 (dichlorine p...
The rate of oxidation of ClO2- by HOCl i...
An aqueous composition includes an activ...
Reactions of aquacobalamin (H2O–Cbl(III)...
Methods and apparatus for generation of ...
The chlorite-periodate reaction has been...
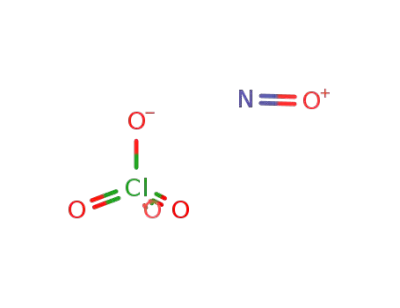
nitrosonium perchlorate

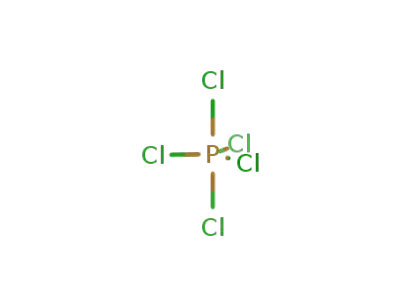
phosphorus pentachloride

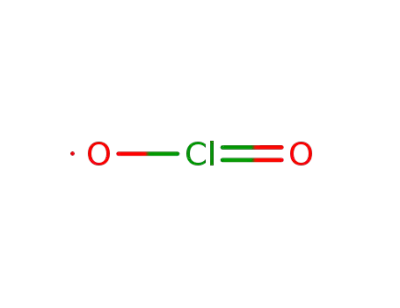
chlorine dioxide

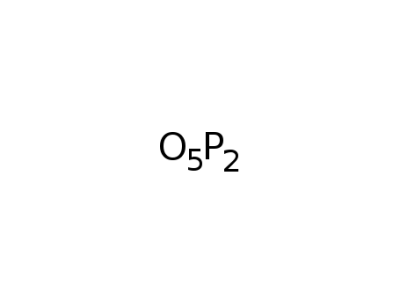
phosphorus pentoxide

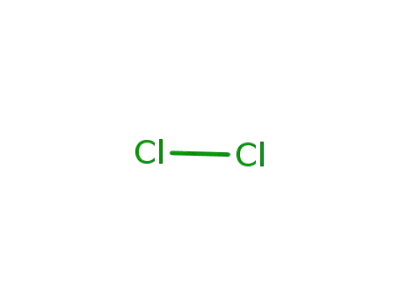
chlorine

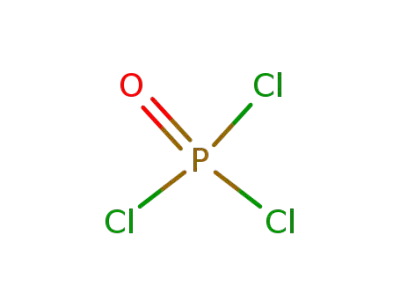
trichlorophosphate
| Conditions | Yield |
|---|---|
|
In
trichlorophosphate;
mixing with NOClO4 in POCl3; decompn.;;
|
|
|
In
neat (no solvent);
mixing with NOClO4; decompn.;;
|
|
|
In
further solvent(s);
mixing with NOClO4 in AsCl3; decompn.;;
|
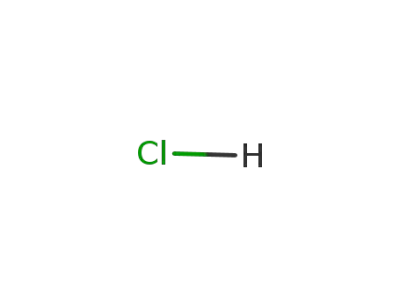
hydrogenchloride

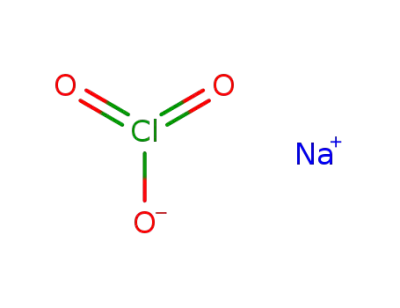
sodium chlorate

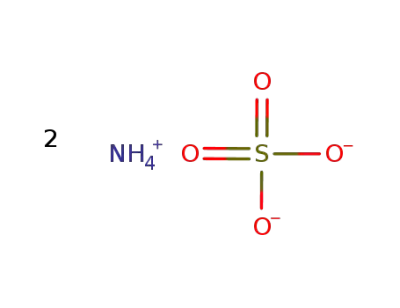
ammonium sulfate

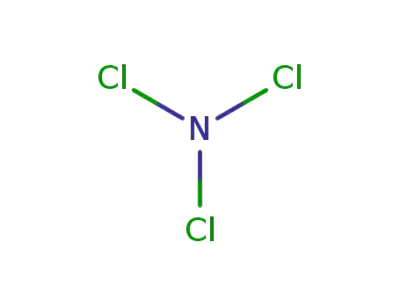
nitrogen trichloride

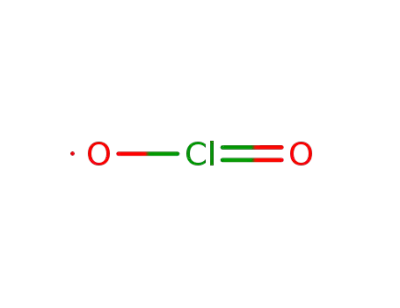
chlorine dioxide
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
|
|
|
In
neat (no solvent);
|
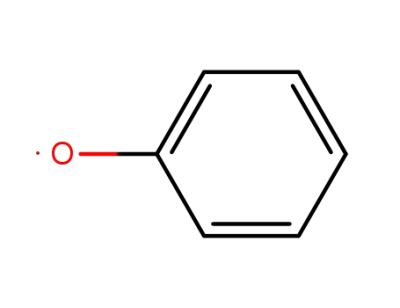
phenoxy radical
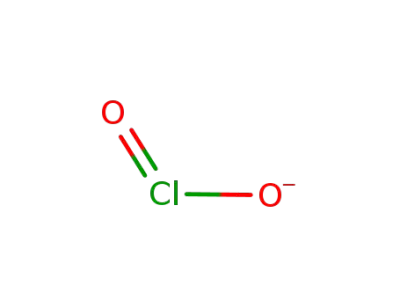
chlorite
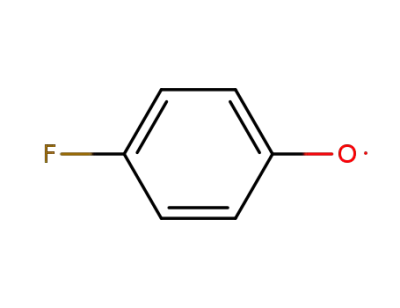
p-fluorophenoxyl radical
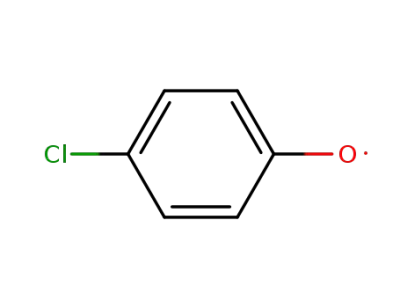
4-chlorophenol radical

p-fluorophenoxyl radical

chlorite
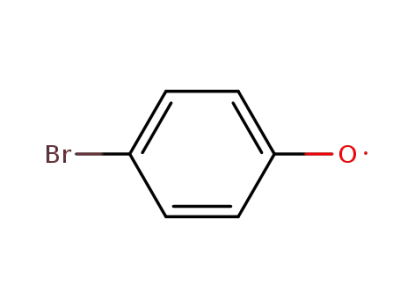
p-Bromophenoxyl radical
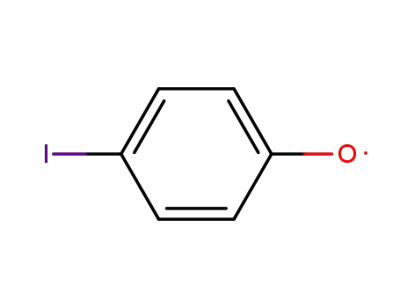
4-iodophenoxyl radical